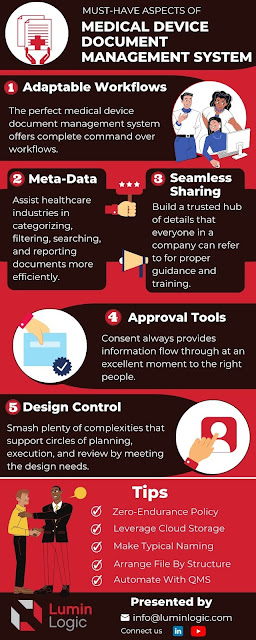
Everything People Need To Know About QMS System In 2022
If people are unknown to the healthcare appliance industry or just ought to touch up on quality system obedience, they likely enclose many queries that start with what, where, and how. A medical apparatus ISO 13485 quality management system protects all facets of a medical device’s biological clock. QMS intends to enhance the grade of medicare devices and relevant favors plus always meet consumer and regulatory needs. Each of these apparatuses points out various conditions for individuals' quality management system (QMS) depending on the complexness of the machines.
Just jump onto the below to understand what QMS is and how it is critical to have this in the medical industry.
QMS in Medical Enterprise:
A healthcare device quality management system is described as a well-defined process that demonstrates the techniques and strategies executed throughout the lifecycle of a medical appliance. As a producer of medicare apparatuses scheduling to market individuals' creations in the global market, they are mandated to incorporate and sustain a QMS deferent with multinational and federal policies plus rules. It is directed as a medical device quality management system.
When Is QMS Mandated?
People may discover it challenging to determine when they should begin a QMS for a healthcare device company. Likewise, the entire time essential to fully form a quality management system can go anywhere between 3 to 9 months. It is even necessary to peek at the demand people are targeting. In the state, individuals must concede with the FDA 21 CFR Part 820 limitations when their medicare device is registered for marketing. Likewise, if a company delivers medium to high-risk healthcare appliances, it will have to get through the 510(K) procedure.
Even though there are massive benefits, it is necessary to have expert guidance for accomplishing it better. Ask for more details!



Comments
Post a Comment